This past week, I was forced to think about the wonderful world of chemistry, for which I thought was something that was set aside many, many years ago.
The hot water heater in the basement, sprang a leak. It was just shy of 3 years old. Initially, we focused on containing and cleaning up the spill. Shut off the water heater and called the plumber that installed the unit.
Jim, the plumber was shocked that the unit went bad so fast. He got a replacement, and they swapped the bad one with a new one, same brand and model. While installing the new one he gave me the name of a company and suggested they come out and check to see if the water softener equipment was working properly, and to have them also test the water.
Sounded like a reasonable suggestion to me, especially after he showed me the incoming water line to the hot water heater which was almost blocked by some kind of substance. There was a lot of buildup inside the coupling, which should not have been there.
I made an appointment the next day, and the suggested company representative showed up. He checked the water softener equipment and stated the equipment appeared to be working correctly. Then, he proceeded to take both hot and cold-water samples from the kitchen sink, and also a sample from the outside spigot. He had hoped that the outside spigot sample would tell us results for water as supplied, before the water softener.
The results were kind of disappointing, in that the meter he used indicated that the outside water spigot was also connected to the water softener, so we did not get to test the pre-treated water supply.
Our water testing showed that the pH was 8.6 and that we had just a trace of Iron in it, plus a there was relatively high level of particulates. He suggested considering adding an under-sink reverse osmosis filter system to remove the particulates and make the water cleaner.
Well, now my scientific brain had a whole lot of data to absorb and process. Chemistry class was pulling me back, and I needed to do research to help me justify what I was thinking.
Scientific thinking can be both a blessing and a curse.
I could just trust this guy, and hope that he wasn’t just trying to sell me something I don’t really need, or, I could try to figure out if there were other potential solutions to the problem at hand.
Me being me, I tend to go with the “now, I will research the heck out of this” approach.
I started by checking the city DPW to determine if they had water test results of the source being distributed to everyone using their water distribution system. The city website had a link to the water department where water test results were posted. The water being sent out for use has a 8.7pH level.
So, back to Chemistry class we go…
Pure water has a pH of 7 at 25°C, meaning it is neutral. When an acid is dissolved in water, the pH will be less than 7, while a base, or alkali, will have a pH greater than 7. A strong acid, such as hydrochloric acid, at concentration 1 mol dm has a pH of 0, while a strong alkali like sodium hydroxide, at the same concentration, has a pH of 14.
A pH scale is used to determine if a liquid is more or less acidic. Acid can be really bad because it causes materials to be eaten away, if it is acidic enough. There are a lot of well know acidic materials, like lye, that will deteriorate materials upon contact. There is also very mild acidic material that are far less damaging, such as citric acid found in many fruits. Many materials can be dissolved in water, thus altering the water pH level.
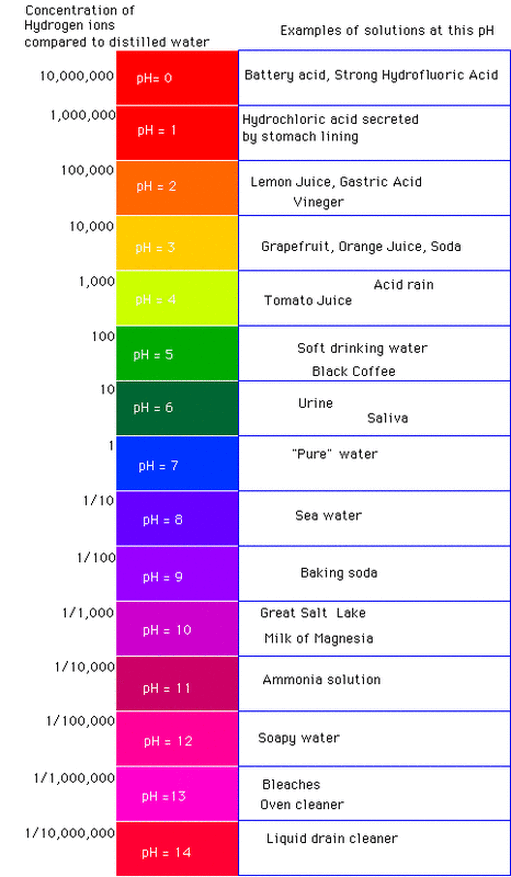
Based on this, it appears that the pH level of our water should be a little bit lower. Keep in mind though that we do have a water softener connected to the incoming water line to the home and the city is providing water at a 8.7pH level.
From what I have determined, the city would rather provide water on the slightly alkaline side rather than on the acidic side of the pH scale. Why?
Because acid causes metals to break down and many older homes used lead pipes. Ever heard of lead poisoning in water – ie: Flint, Michigan, which is about 40 minutes away from where we live?
Okay, so if there are side effects for too much acid in water, what is the side effect of too much alkaline in the water?
Scaling is one side effect. This is a residue that leaves a white chalkiness on things after the water droplets dry up. The stuff that is found on water faucets and shower heads in the bathroom, and on the glasses in the dish washer. Most likely some combination of sulfates, calcites, or sodium in the water.
Science always adds little twists too. Notice the pH scale definition included a temperature (at 25 degrees Celsius). This means that the pH level of materials changes when the temperature goes up or down.
Assuming the Hot water heater will impact the pH level, which way will the pH level go as water is heated? Not surprisingly, it is up. So, the scaling caused by the slightly high pH will actually be accelerated by the heating it. Well, that helps explain a few things.
There are a few ways available to lower the pH level of water. CO2 injectors will potentially do that by converting certain ions with existing materials in the water. Another method is to introduce a material that is slightly more acidic to reduce the pH level. There is also a method used to pass the water through a magnetic or electrical field, causing certain ions to be displaced, and change the chemical make-up of whatever is in the water flowing through the pipes.
I found that there are a few citric acid filters that can be used to do just that and will also act as a descaler of the existing build up in the water lines. Our home was built using copper pipes, so no worries about lead poisoning. I am considering adding a citric acid type filter to the cold-water pipe between the water softener and the hot water heater. This would be a way to reduce the scale build up inside the hot water heater. Scale build-up inside the hot water heater can contribute to a higher energy bill, because it takes more energy to heat the water with scale inside of it. And, it is said that it contributes to the water heater failing sooner than it would with less scale build-up.
Interestingly enough, the guy that tested the water and checked the water softener just emailed me and suggested adding a citric acid-based unit to the water system. His company could install one for about $2,000 and it would handle all the water in the entire home. We haven’t responded to that proposal yet. I am concerned about the cost to replace that unit it when it wears out in 1o years, and the cost of the filters when it is time to replace them. More for discussion next week when we discuss this.
So, I am glad to know that they think the water softener is doing its job, based on the lack of hard metals and certain minerals were not present in the water samples. I am glad that I now have a better understanding of why the 3-year-old hot water heater may have failed prematurely.
Now, we can move forward and use that knowledge to make a better-informed decision on what to do to reduce or hopefully eliminate the scale build up on the fixtures and equipment in the home.
In any case, once again, Science comes to the rescue. I love science!

So your neighbors should be planning on buying new water heaters soon? I think I would be upset.Sent via the Samsung Galaxy S8+, an AT&T 5G Evolution capable smartphone
LikeLike
Yeah Jan, so here is the weird thing about it – one neighbor 2 doors down has replaced 2 water heaters in 4 years, while another has had the same one for 14 years. We are all using the same water supply from the same source and from the same pipeline. I don’t get it, I guess some have just been luckier than others.
LikeLike